CHM 108: Chemistry Practical Manual
Department of Chemical Sciences – 2024/2025 Academic Session
Overview
This manual contains detailed practical experiments for CHM 108 including organic and analytical chemistry techniques such as fractional crystallization, chromatography, hydrocarbon testing, functional group identification, and salt analysis. Each section is tailored to prepare students with hands-on lab experience and proper documentation skills.
Practical 1: Separation by Fractional Crystallization
Prelab Questions
- Why is CuSO₄ more soluble in water than in organic solvent?
- Why does solubility of solids increase with temperature?
- Evaluate experimental errors in salicylic acid and copper sulfate recovery.
Experimental Summary
Mixture of salicylic acid and CuSO₄·5H₂O separated using solubility and cooling techniques to yield solid crystals. Analysis includes mass calculation and percentage recovery.
Practical 2: Separation Using Chromatography
Prelab Questions
- Why do some substances travel further on chromatograms?
- How can chromatography confirm aspirin synthesis?
- Define: Moving Phase, Adsorption
Experimental Summary
Thin-layer chromatography of chloroplast extracts with measurement of Rf values. Students prepare chromatograms and record color band movement and separation.
Practical 3: Physical Properties of Hydrocarbons
Prelab Questions
- Draw structural formulas: ethane, toluene, propane
- Define: Hydrocarbon, Alkane, Alkene, Alkyne, Aromatic Hydrocarbon
Observations
- Odor and water solubility of hexane, 1-pentene, and toluene
- Solubility of hydrocarbons in each other
- General statement about hydrocarbon solubility
Practical 4: Chemical Properties of Hydrocarbons
Tests Included
- Reaction with bromine (substitution/addition)
- Potassium permanganate test for unsaturation
- Evaluation of kerosene and unknown hydrocarbons
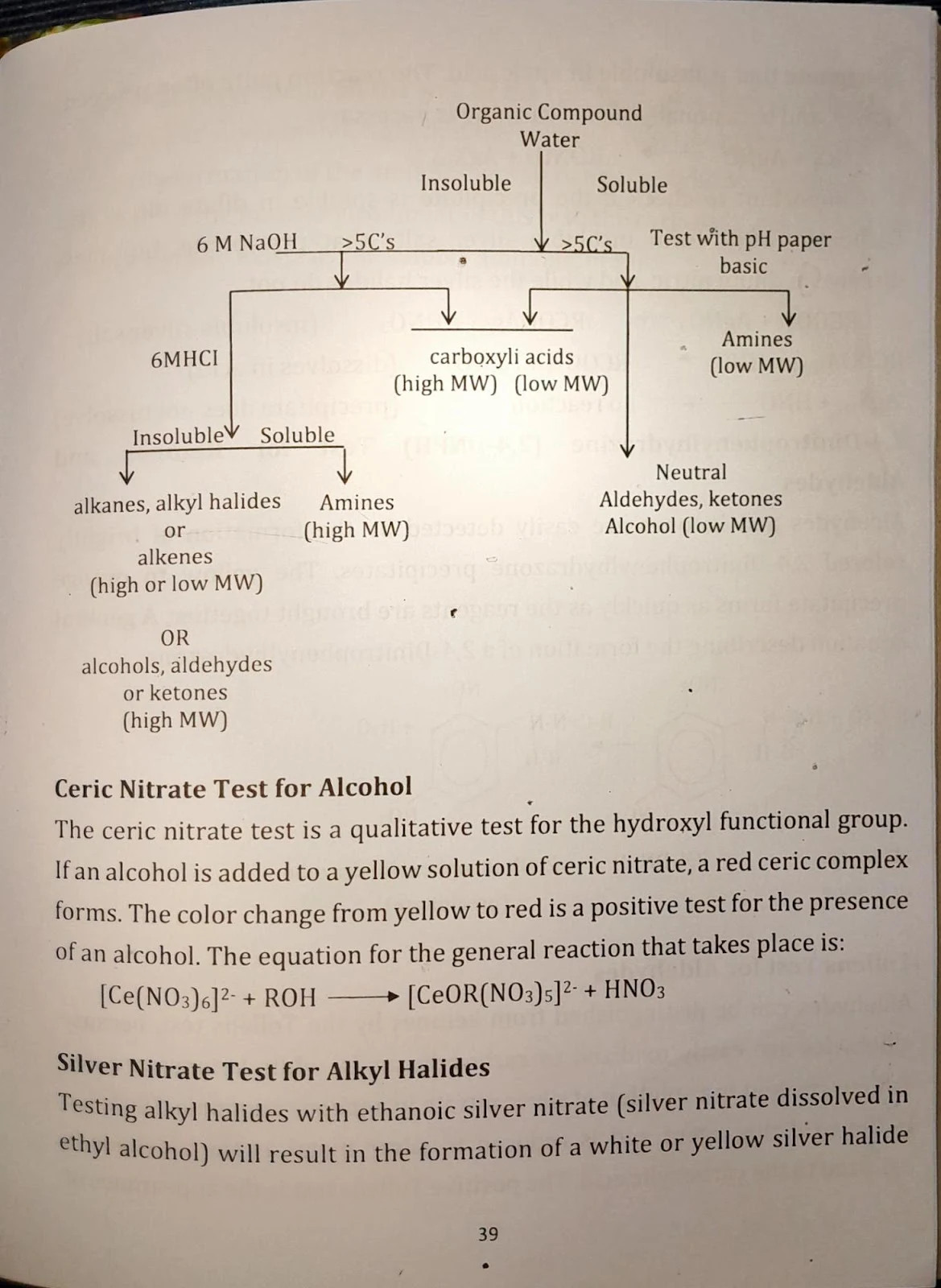
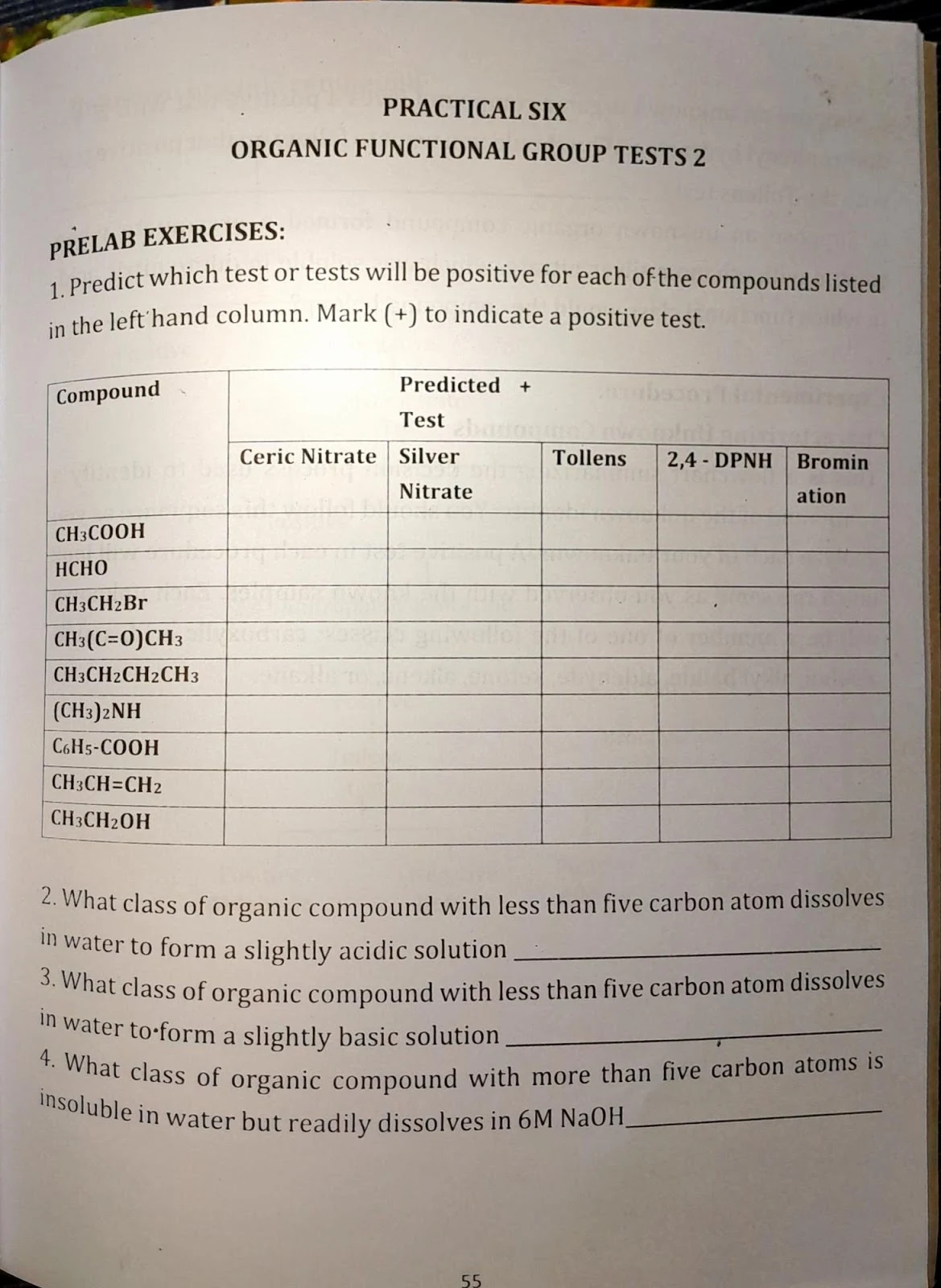
Practical 5 & 6: Functional Group Tests (1 & 2)
Tests Conducted
- Solubility tests with water, NaOH, and HCl
- Ceric nitrate test for alcohols
- Silver nitrate test for halides
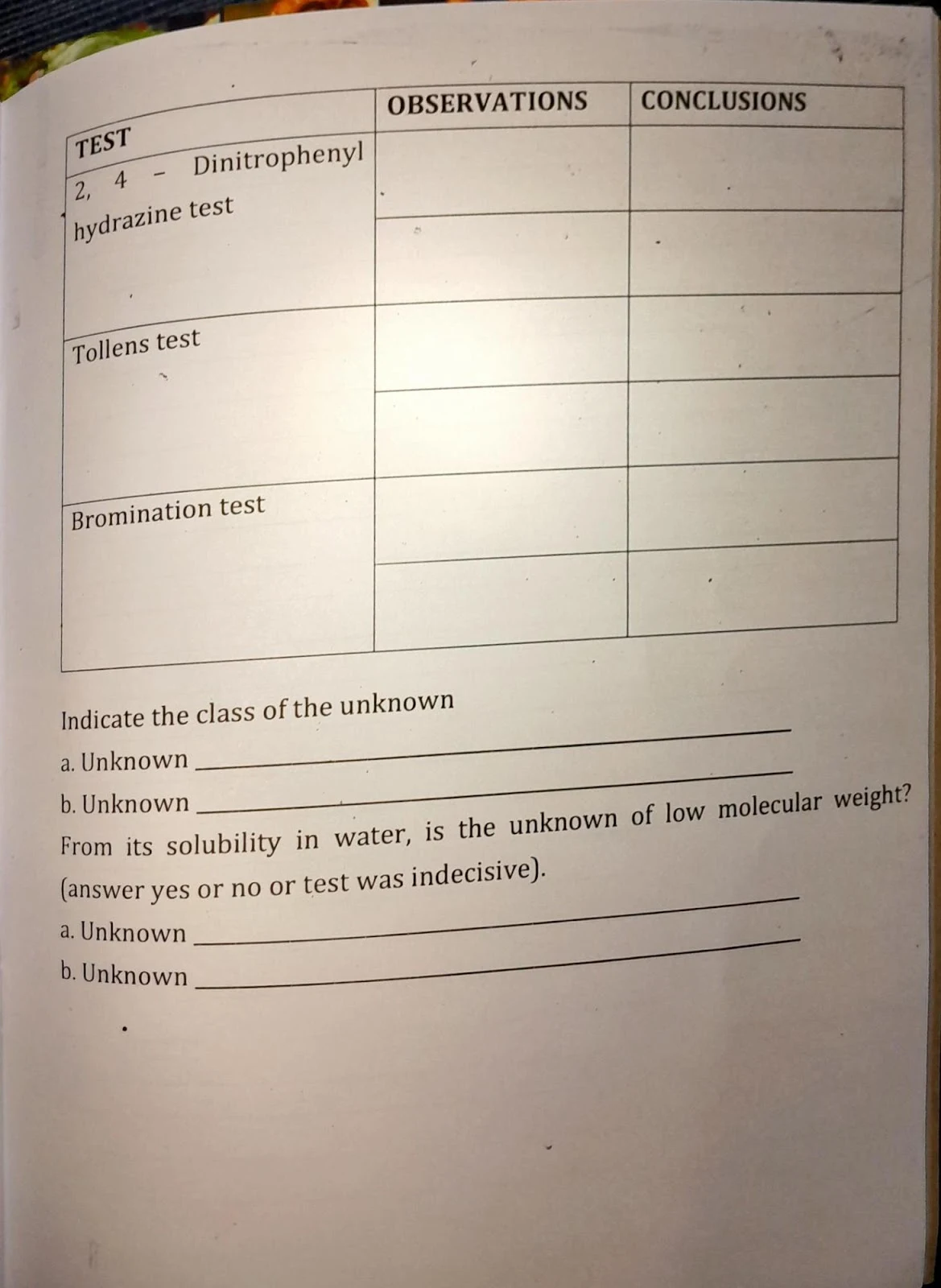
- 2,4-DNPH test for aldehydes & ketones
- Tollens test for aldehydes
- Bromination test for alkenes
Analysis and conclusion based on positive or negative results from each functional group test.
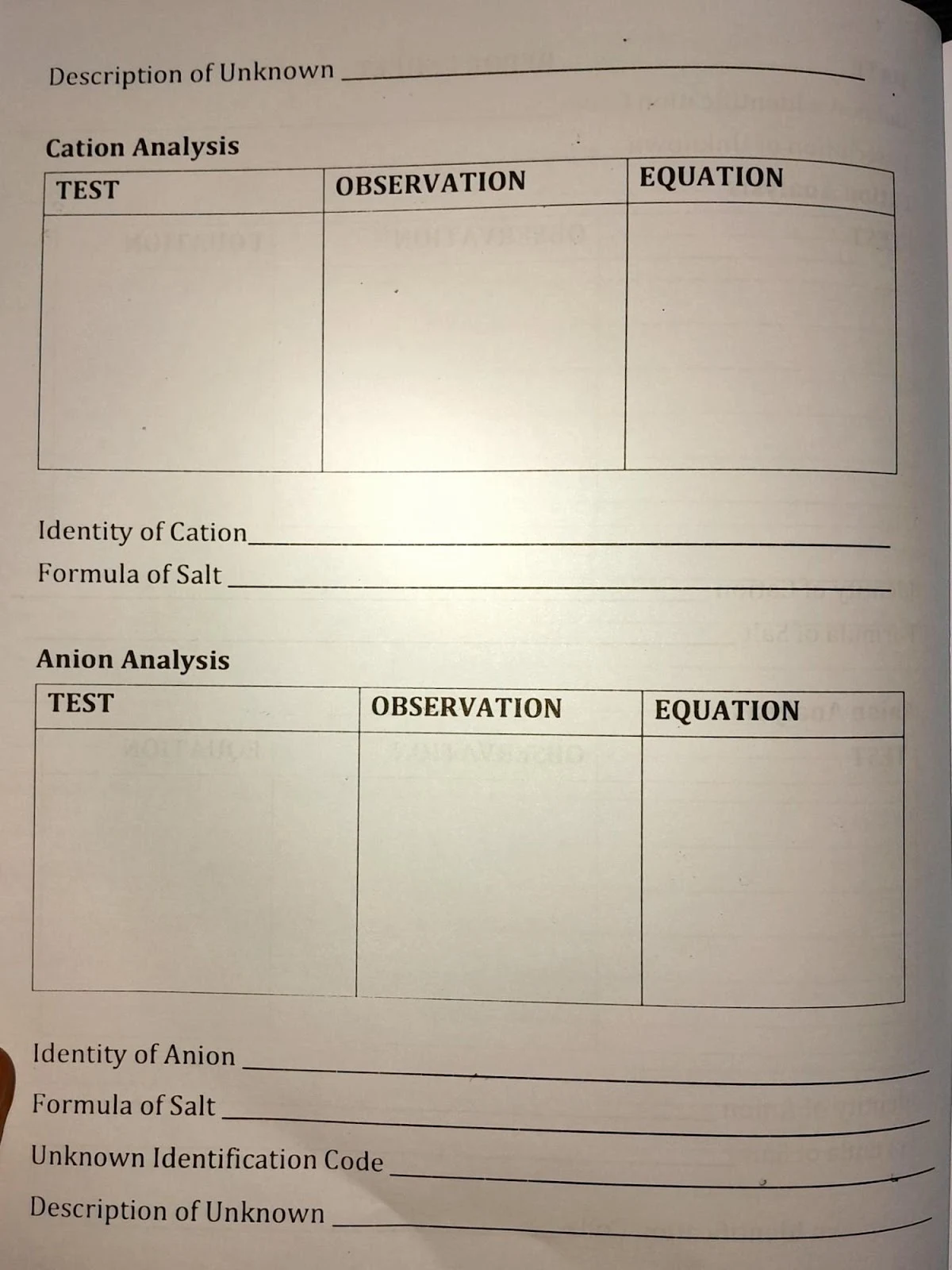
Practical 7: Analysis of Soluble Salts
Cation & Anion Analysis
- Flame test and litmus observation for cations (Na⁺, K⁺, NH₄⁺, Cu²⁺, Ni²⁺, Ba²⁺)
- Use of silver nitrate, nitric acid, and BaCl₂ to identify common anions (Cl⁻, I⁻, CO₃²⁻, SO₄²⁻, CrO₄²⁻)
- Identification of salts based on solubility rules and precipitation tests

























































0 Comments